Research Overview
Team Members
We are always looking for motivated individuals interested in our research. If you feel you are qualified do not hesitate to contact us.
To contact any current member simply click on their image.
Lab Mascot/Guardian
Onyx
Postdoctoral Scholars
Doctoral Students

Academia Copernicana
Interdisciplinary Doctoral School
Zainab Kazim

Academia Copernicana
Interdisciplinary Doctoral School
Weronika Nowak
Master's Students

Biotechnology
Katarzyna Świstowska

Biotechnology
Marcelina Mikołajczyk

Biotechnology
Magda Tylenda

Biotechnology
Kuba Woliński

Biotechnology
Zuzanna Szutkowska
Bachelor Students
Former Members
Master's Students
Kamila
Grygiel

Bachelor Students
Agata
Wikarska

Bachelor Students
Kacper
Roszak

Bachelor Students
Jakub
Lewandowski
Projects
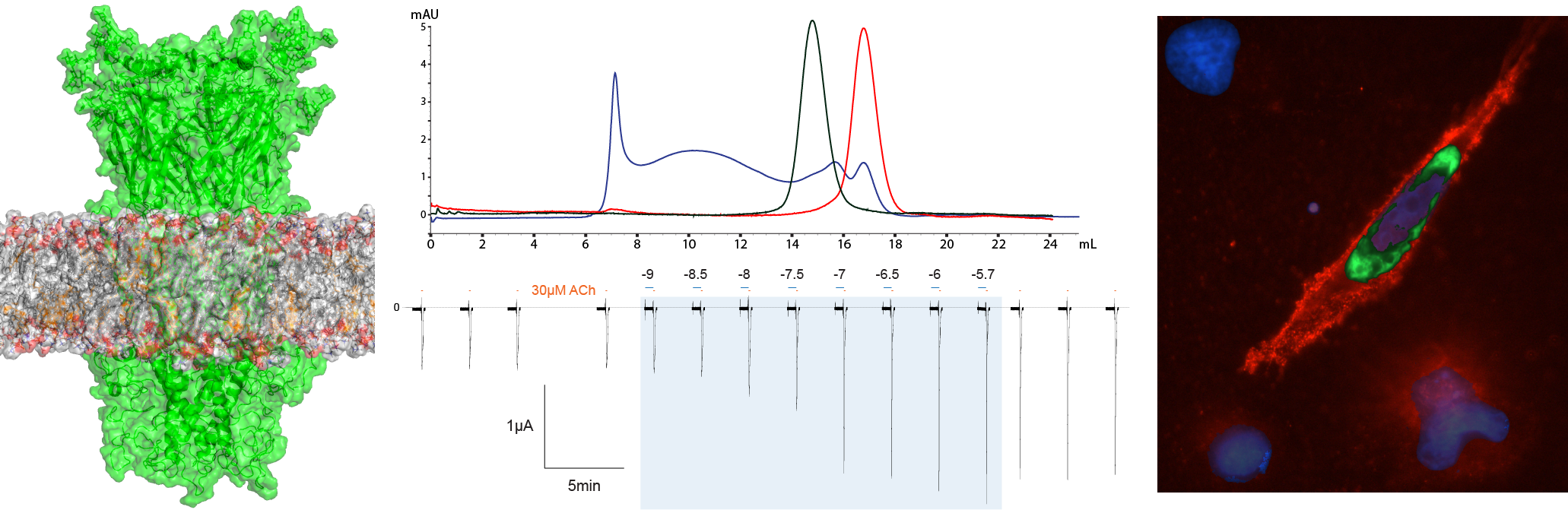
Combating addiction by targeting α3β4*-nicotinic acetylcholine receptors
OPUS21-2021/41/B/NZ7/03101
According to the World Health Organization tobacco use kills more than 7 million people every year and costs an estimate of US1.4 trillion in global economic burden resulting from loss of productivity and healthcare costs. Smoking addiction has long been linked to the cholinergic system, specifically nicotinic acetylcholine receptors, through the principal addictive component in cigarettes: nicotine. These receptors bind a chemical signal (neurotransmitters) and convert it to an electrical signal (ion conductance). They belong to a class of ligand-gated ion channels composed of five individual similar or sometimes identical protein subunits that form an ion channel, otherwise known as pentameric ligand-gated ion channels. This family of receptors is involved in all major functions of the central nervous system. Numerous years of pharmacological and therapeutic research targeting the neurotransmitter and modulatory binding sites of nicotinic acetylcholine receptors has not yet produced any robust anti-addiction therapies. Recent genomic studies have identified a specific gene cluster and therefore the α3-, β4-, α5-subunits of the nicotinic acetylcholine receptor family which are correlated to smoking addiction. An inhibition of this assembly of nicotinic receptor subunits has been shown to reduce the reward effect caused by drug interactions in the brain, and thereby attenuate nicotine as well as other drug addictions.
This project expressly aims to synthetically generate and isolate selective single-domain antibodies which act as inhibitory modulators of α3-β4 nicotinic acetylcholine receptor subunits. These small versatile antibodies are derived from camelids such as llamas, alpacas, camels, and dromedaries. Very recently it has been possible to create synthetic libraries of single-domain antibodies and thereby avoid the use of camelids to generate them. These synthetically generated and selective nanobodies will also be used as tools to properly localize various α3β4 receptor assemblies in the brain, thereby better understanding the role of they play in the brain’s reward pathway.
Understanding the role nicotinic acetylcholine receptors play in addiction is an important aspect to developing efficient therapeutics. This proposal attempts to develop therapeutics to help alleviate the drug dependence of smoking and other drug addictions, thereby reducing the economic burden that cancer and other addiction-related diseases have on the healthcare system. The use of single-domain antibodies as tools to study receptor localization will also elaborate upon the current understanding of the addiction/reward pathway in the brain.
This project expressly aims to synthetically generate and isolate selective single-domain antibodies which act as inhibitory modulators of α3-β4 nicotinic acetylcholine receptor subunits. These small versatile antibodies are derived from camelids such as llamas, alpacas, camels, and dromedaries. Very recently it has been possible to create synthetic libraries of single-domain antibodies and thereby avoid the use of camelids to generate them. These synthetically generated and selective nanobodies will also be used as tools to properly localize various α3β4 receptor assemblies in the brain, thereby better understanding the role of they play in the brain’s reward pathway.
Understanding the role nicotinic acetylcholine receptors play in addiction is an important aspect to developing efficient therapeutics. This proposal attempts to develop therapeutics to help alleviate the drug dependence of smoking and other drug addictions, thereby reducing the economic burden that cancer and other addiction-related diseases have on the healthcare system. The use of single-domain antibodies as tools to study receptor localization will also elaborate upon the current understanding of the addiction/reward pathway in the brain.
Past Projects
Investigation into the mechanism of regulation of nicotinic acetylcholine receptors
POLS-2020/37/K/NZ3/04098
Neurological disorders such as Alzheimer’s disease, schizophrenia, and Parkinson’s disease, in addition to drug addiction have long been linked to the cholinergic system, and specifically nicotinic acetylcholine receptors. These receptors bind a chemical signal (neurotransmitters) and convert it to an electrical signal (ion conductance). They belong to a class of pentameric ligand-gated ion channels composed of five individual similar or sometimes identical protein subunits that form an ion channel. They have a conserved general structure which includes three domains. The extracellular domain, the protein region located outside of the cell, contains the neurotransmitter binding site. The transmembrane domain forms the ion channel pore that selectively allows ions to flow along their concentration gradient. The intracellular domain, the protein region inside of the cell, is thought to be primarily involved in the regulation and trafficking of the receptor. This family of receptors is involved in all major functions of the central nervous system. An enhanced understanding of the mechanisms for functional modulation, in addition to the structural determination of the neurotransmitter binding site, resulted in many pharmacological advances. Yet the numerous years of research have not produced any robust therapies for the maledictions caused by a dysfunction of nicotinic acetylcholine receptors.
This project expressly aims to study the mechanism(s) of functional regulation of nicotinic acetylcholine receptors through their intracellular domain, for which very little is known. Targeting the regulatory system of these receptors may form the basis for developing new therapeutics against neurological disorders.
Within the project a protein composed of an intracellular domain of nicotinic acetylcholine receptors linked to a soluble homologous protein will be created. Using this soluble linked-protein as a tool to identify proteins which interact with the intracellular domain, this project will determine novel targets for current pharmaceutical therapeutic objectives. These newly discovered targets will generate more successful remedies for neurological disorders such as Alzheimer’s and schizophrenia.
Additionally this project will study the mechanisms of action of the identified regulatory proteins. The subtype selective regulation may be identified by studying the role that the various subunit compositions of the receptor, otherwise known as stoichiometry, play in regulatory protein binding. Understanding the intricate mechanism of receptor regulation is important to combat neurodegenerative diseases. Through the development of small single-domain antibodies, nanobodies, against specific receptor stoichiometries this project will answer questions about regulatory differences and develop an understanding of regulatory mechanisms. These same nanobodies will also be used in the future projects as tools to properly localize given stoichiometries in the brain, creating a translational bridge between the biochemical mechanisms of regulation to the neurobiological system composition.
Understanding the mechanisms of regulation of nicotinic acetylcholine receptors, an aspect that has thus far remained elusive, is the key to developing efficient therapeutics for neurological disorders. This proposal attempts to develop such an understanding in the hope that a more effective forthcoming pharmaceutical approach may arise as a result.
This project expressly aims to study the mechanism(s) of functional regulation of nicotinic acetylcholine receptors through their intracellular domain, for which very little is known. Targeting the regulatory system of these receptors may form the basis for developing new therapeutics against neurological disorders.
Within the project a protein composed of an intracellular domain of nicotinic acetylcholine receptors linked to a soluble homologous protein will be created. Using this soluble linked-protein as a tool to identify proteins which interact with the intracellular domain, this project will determine novel targets for current pharmaceutical therapeutic objectives. These newly discovered targets will generate more successful remedies for neurological disorders such as Alzheimer’s and schizophrenia.
Additionally this project will study the mechanisms of action of the identified regulatory proteins. The subtype selective regulation may be identified by studying the role that the various subunit compositions of the receptor, otherwise known as stoichiometry, play in regulatory protein binding. Understanding the intricate mechanism of receptor regulation is important to combat neurodegenerative diseases. Through the development of small single-domain antibodies, nanobodies, against specific receptor stoichiometries this project will answer questions about regulatory differences and develop an understanding of regulatory mechanisms. These same nanobodies will also be used in the future projects as tools to properly localize given stoichiometries in the brain, creating a translational bridge between the biochemical mechanisms of regulation to the neurobiological system composition.
Understanding the mechanisms of regulation of nicotinic acetylcholine receptors, an aspect that has thus far remained elusive, is the key to developing efficient therapeutics for neurological disorders. This proposal attempts to develop such an understanding in the hope that a more effective forthcoming pharmaceutical approach may arise as a result.
Lab Anouncements
- The Nemecz Lab has an open Post-doctoral Fellowship position:
Post-Doctoral Position Announcement within NCN OPUS-21 Project
Uniwersytet Mikołaja Kopernika w Toruniu/Nicolaus Copernicus University in Toruń
Start Date: As soon as possible
Funding: 10,000 PLN/month (gross/gross: employer taxes, pension, health insurance and income taxes are deducted from this amount ≈7608 PLN/month [gross: personal income tax and social security deducted from this amount] with 13th month)
Duration: 12month contract
Candidatures will be evaluated on a rolling basis, until the position is filled.
Project Description: Generation and characterization of synthetic single domain VHH antibodies against nicotinic acetylcholine receptor subtypes. Localization of these subtypes in the brain tissue samples. Under the supervision of the project leader: Dr. Ákos Nemecz. More information about the lab and projects may be found at: nemeczlab.umk.pl
Requirements:
- Ph.D. in biochemistry, pharmacology, biology, or related field.
(PhD degree must be awarded by another institution than UMK w Toruniu or a continuous post-doctoral fellowship of at least 10 months has been completed, in another country than Poland, prior to hire); - Ability to work independently on a scientific project including: planning, completing, analyzing experiments as well as the ability to properly present the results;
- Fluent in written and spoken English.
- Experience in protein purification techniques and immunostaining
- Good communication and team working skills
- A cover/motivation letter
- CV, including list of publications/abstracts
- A list of contact information (name, email, position/institution) for three references included in either the CV or cover letter
- A signed GDPR statement: Found here
- Ph.D. in biochemistry, pharmacology, biology, or related field.
- 2025-10-27: Congratulations to Katarzyna Świstowska for obtaining the Student Scholarship position in our lab.
- 2025-11-(15–19): Weronika Nowak will present an abstract at the SFN annual meeting in San-Diego, USA.
- 2025-10-(11-14): Weronika Nowak will present an abstract at the 38th ECNP Congress in Amsterdam, the Netherlands.
- 2025-09-11:Congratulations on a successfull defense MSc Katarzyna Świstowska!
- 2025-06-(16-19): Katarzyna Świstowska will present an abstract at the FENS Regional Meeting in Oslo, Norway.
- 2025-06-04: Weronika Nowak obtained a training course scholarship as part of the “Excellence Initiative-Research University”.
- 2025-05-(5-8): Katarzyna Świstowska will present an abstract at the EMBO Workshop: Cell Biology of the Nervous System: Cellular Mechanisms of Communication in Herakion, Greece.
- 2025-03-20: Katarzyna Świstowska won a Grants4NCU conference travel grant to attend the FENS Regional Meeting 2025.
- 2025-03-20: Weronika Nowak won a Grants4NCU conference travel grant to attend the 38th ECNP Congress.
- 2024-12-12: Kuba Woliński (PI) and Weronika Nowak won a Grants4NCU group project entitled: Modification and Analysis of Specificity and Selectivity of VHH Sequences Targeting the α4β2 Nicotinic Acetylcholine Receptor (nAChR).
- 2024-10-01: Weronika Nowak begins her doctoral studies with Academia Copernicana
Publications
Scroll to Change
- All Publications
- Highlighted Publications
- Dr. Ákos Nemecz
- dr Dorota Nemecz
- All Publications
- Nemecz D.§, Nowak W.A., Nemecz Á.§ “VHH nanobody versatility against pentameric ligand-gated ion channels.” J Med Chem (2024-06-03); 67(11):8502-8518. DOI: 10.1021/acs.jmedchem.4c002316
- Van Renterghem C.§, Nemecz Á., Medjebeur K., Corringer P-J.§ “Short-chain mono-carboxylates as negative modulators of allosteric transitions in Gloeobacter violaceus ligand-gated ion channel, and impact of a pre-β5 strand (Loop Ω) double mutation on crotonate, not butyrate effect.” Physiol Rep (2024-02-11); 12(3):e15916. DOI: 10.14814/phy2.15916
- Van Renterghem C.§, Nemecz Á., Delarue-Cochin S., Joseph D., Corringer P-J.§ “Fumarate as positive modulator of allosteric transitions in the pentameric ligand-gated ion channel GLIC: Requirement of an intact vestibular pocket.” J Physiol (2023-06); 601(12):2447-2472. DOI: 10.1113/JP283765. Epub 2023-04-27
- Li Q., Nemecz Á.§, Aymé G.§, Dejean de la Bâtie G., Prevost M.S., Pons S., Barilone N., Baachaoui R., Maskos U., Lafaye P., Corringer P-J.§ “Generation of nanobodies acting as silent and positive allosteric modulators of the α7 nicotinic acetylcholine receptor.” Cell Mol Life Sci (2023-05-25); 80(6):164. DOI: 10.1007/s00018-023-04779-8
- Nemecz D.§, Golińska P. “Antibacterial Properties of Crotoxin from Crotalus durissus terrificus-Insight into the Mechanism of Action.” Molecules (2022-11-10); 27(22):7726. DOI: 10.3390/molecules27227726
- Nemecz D.*, Ostrowski M.*, Ravatin M., Saul F., Faure G.§ “Crystal Structure of Isoform CBd of the Basic Phospholipase A2 Subunit of Crotoxin: Description of the Structural Framework of CB for Interaction with Protein Targets.” Molecules (2020-11-13); 25(22):5290. DOI: 10.3390/molecules25225290
- Hu H.*, Nemecz Á.*, Fourati Z., Van Renterghem C., Sauguet L., Corringer P-J., Delarue M.§ “Crystal structures of a pentameric ion channel gated by alkaline pH show a widely open pore and identify a cavity for modulation.” Proc Natl Acad Sci U S A (2018-04-24); 115(17):E3959-E3968. DOI: 10.1073/pnas.1717700115
- Bajek A., Olkowska J., Walentowicz-Sadłecka M., Sadłecki P., Grabiec M., Porowińska D., Drewa T., Roszkowski K.§ “Human Adipose-Derived and Amniotic Fluid-Derived Stem Cells: A Preliminary In Vitro Study Comparing Myogenic Differentiation Capability.” Med Sci Monit. (2018-03-24); 24:1733-1741. DOI: 10.12659/msm.905826
- Nemecz Á., Hu H., Fourati Z., Van Renterghem C., Delarue M., Corringer P-J.§ “Full mutational mapping of titratable residues helps to identify proton-sensors involved in the control of channel gating in the Gloeobacter violaceus pentameric ligand-gated ion channel.” PLoS Biol (2017-12-27); 15(12):e2004470. DOI: 10.1371/journal.pbio.2004470
- Maj M.*§, Bajek A.*, Nalejska E., Porowińska D., Kloskowski T., Gackowska L., Drewa T. “Influence of Mesenchymal Stem Cells Conditioned Media on Proliferation of Urinary Tract Cancer Cell Lines and Their Sensitivity to Ciprofloxacin.” J Cell Biochem. (2017-06); 118(6):1361-1368. DOI: 10.1002/jcb.25794. Epub 2016-11-30.
- Wujak M., Hetmann A., Porowińska D., Roszek K.§ “Gene Expression and Activity Profiling Reveal a Significant Contribution of Exo-Phosphotransferases to the Extracellular Nucleotides Metabolism in HUVEC Endothelial Cells.” J Cell Biochem. (2017-06); 118(6):1341-1348. DOI: 10.1002/jcb.25791. Epub 2017-01-03.
- Czarnecka J., Porowińska D., Bajek A., Hołysz M., Roszek K.§ “Neurogenic Differentiation of Mesenchymal Stem Cells Induces Alterations in Extracellular Nucleotides Metabolism.” J Cell Biochem. (2017-03); 118(3):478-486. DOI: 10.1002/jcb.25664. Epub 2016-08-25.
- Bajek A.§, Olkowska J., Walentowicz-Sadłecka M., Walentowicz P., Sadłecki P., Grabiec M., Bodnar M., Marszałek A., Dębski R., Porowińska D., Czarnecka J., Kaźmierski Ł., Drewa T. “High Quality Independent From a Donor: Human Amniotic Fluid Derived Stem Cells-A Practical Analysis Based on 165 Clinical Cases.” J Cell Biochem. (2017-01); 118(1):116-126. DOI: 10.1002/jcb.25618. Epub 2016-06-28.
- Roszek K.§, Makowska N., Czarnecka J., Porowińska D., Dąbrowski M., Danielewska J., Nowak W. “Canine Adipose-Derived Stem Cells: Purinergic Characterization and Neurogenic Potential for Therapeutic Applications.” J Cell Biochem. (2017-01); 118(1):58-65. DOI: 10.1002/jcb.25610. Epub 2016-06-03.
- Ostrowski M.§, Mierek-Adamska A., Porowińska D., Goc A., Jakubowska A. “Cloning and biochemical characterization of indole-3-acetic acid-amino acid synthetase PsGH3 from pea.” Plant Physiol Biochem. (2016-10); 107:9-20. DOI: 10.1016/j.plaphy.2016.05.031. Epub 2016-05-20.
- Ostrowski M., Porowińska D., Prochnicki T., Prevost M., Raynal B., Baron B., Sauguet L., Corringer P-J., Faure G.§ “Neurotoxic phospholipase A2 from rattlesnake as a new ligand and new regulator of prokaryotic receptor GLIC (proton-gated ion channel from G. violaceus).” Toxicon (2016-06-15); 116:63-71. DOI: 10.1016/j.toxicon.2016.02.002. Epub 2016-02-05.
- Nemecz Á.*, Prevost, M.S.*, Menny A.*, Corringer P-J.§ “Emerging Molecular Mechanisms of Signal Transduction in Pentameric Ligand-Gated Ion Channels.” Neuron (2016-05-04); 90(3):452-70. Review. DOI: 10.1016/j.neuron.2016.03.032
- Roszek K.§, Porowińska D., Bajek A., Hołysz M., Czarnecka J. “Chondrogenic Differentiation of Human Mesenchymal Stem Cells Results in Substantial Changes of Ecto-Nucleotides Metabolism.” J Cell Biochem. (2015-12); 116(12):2915-23. DOI: 10.1002/jcb.25239
- Bajek A.*§, Porowińska D.*, Kloskowski T., Brzoska E., Ciemerych MA., Drewa T. “Cell therapy in Duchenne muscular dystrophy treatment: clinical trials overview.” Crit Rev Eukaryot Gene Expr. (2015); 25(1):1-11. DOI: 10.1615/critreveukaryotgeneexpr.2015011074
- Stadnicka K., Marszałek A., Kozłowska I., Walasik K., Bodnar M., Bajek A., Porowińska D., Drewa T., Bednarczyk M. “The expression of p63 and Ck HMW in magnum and infundibulum of Gallus domesticus oviduct.” Folia Biol (Krakow) (2014); 62(3):179-85. DOI: 10.3409/fb62_3.179
- Sauguet L.*, Shahsavar A.*, Poitevin F.*, Huon C., Menny A., Nemecz Á., Haouz A., Changeux J-P, Corringer P-J, Delarue M.§ “Crystal structures of a pentameric ligand-gated ion channel provide a mechanism for activation.” Proc Natl Acad Sci U S A (2014-01-21); 111(3):966-71. DOI: 10.1073/pnas.1314997111
- Uzarska M.§, Porowińska D., Bajek A., Drewa T. “[Epidermal stem cells--biology and potential applications in regenerative medicine].” Postepy Biochem. (2013); 59(2):219-27.
- Wujak M., Banach M., Porowińska D., Piskulak K., Komoszyński M.§ “Isolation and bioinformatic analysis of seven genes encoding potato apyrase. Bacterial overexpresssion, refolding and initial kinetic studies on some recombinant potato apyrases.” Phytochemistry (2013-09); 93:8-17. DOI: 10.1016/j.phytochem.2013.03.014. Epub 2013-05-09.
- Porowińska D.§, Wujak M., Roszek K., Komoszyński M. “[Prokaryotic expression systems].” Postepy Hig Med Dosw (Online) (2013-03-01); 67:119-29. DOI: 10.5604/17322693.1038351
- Yamauchi J.G., Gomez K., Grimster N.P., Dufoil M., Nemecz Á., Fotsing J.R, Ho K-Y., Talley T.T., Sharpless K.B., Fokin V.V, Taylor P.§ “Synthesizing selective agonists for the α7 nicotinic receptor with in situ click-chemistry on acetylcholine binding protein templates.” Mol. Pharmacol. (2012-10); 82(4):687-99. DOI: 10.1124/mol.112.080291
- Rojsanga P., Boonyarat C., Utsintong M., Nemecz Á., Yamauchi J.G., Talley T.T., Olson A.J., Matsumoto K., Vajragupta O.§ “The effect of crebanine on memory and cognition impairment via the alpha-7 nicotinic acetylcholine receptor.” Life Sci. (2012-08-21); 91(3-4):107-14. DOI: 10.1016/j.lfs.2012.06.017
- Porowińska D., Marszałek E., Wardęcka P., Komoszyński M.§ “[In vitro renaturation of proteins from inclusion bodies].” Postepy Hig Med Dosw (Online) (2012-06-11); 66:322-9. DOI: 10.5604/17322693.999918
- Grimster N.P., Stump B., Fotsing J.R., Weide T., Talley T.T., Yamauchi J.G., Nemecz Á., Kim C., Ho K-Y., Sharpless K.B., Taylor P., Fokin, V.V.§ “Generation of candidate ligands for nicotinic acetylcholine receptors via in situ click chemistry with a soluble acetylcholine binding protein template.” J Am Chem Soc (2012-04-18); 134(15):6732-40. DOI: 10.1021/ja3001858
- Nemecz Á., Taylor P.§ “Creating an α7 nicotinic acetylcholine recognition domain from the acetylcholine binding protein: crystallographic and ligand selectivity analysis.” J Biol Chem (2011-12-09); 286(49):42555-65. DOI: 10.1074/jbc.M111.286583
- Yamauchi J.G., Nemecz Á., Nguyen Q.T., Muller A., Schroeder L.F., Talley T.T., Lindstrom J., Kleinfeld D., Taylor P.§ “Characterizing ligand-gated ion channel receptors with genetically encoded Ca2++ sensors.” PLoS One (2011); 6(1):e16519. DOI: 10.1371/journal.pone.0016519
- Porowińska D., Czarnecka J., Komoszyński M.§ “[The role of ectonucleotides metabolizing enzymes in purinergic signaling].” Postepy Biochem. (2011); 57(3):294-303.
2024
2023
2022
2020
2018
2017
2016
2015
2014
2013
2012
2011
Current Funding

Previous Funding







